
Unlock the Full Potential of Your Proteomics Research with

Accelerate discovery with Bioinformatics Solutions Inc (BSI) —your partner in mass spectrometry-driven proteomics, immunopeptidomics, glycoproteomics, and pharmaceutical research. Our cutting-edge PEAKS software and in-house MS expertise provide the precision and depth needed for confident, data-driven decisions.
With high-efficiency workflows, rapid turnaround times, and expert PhD support, we deliver high-quality insights to advance drug discovery, biomarker research, and therapeutic development.
Connect with BSI and push the boundaries of biological research.
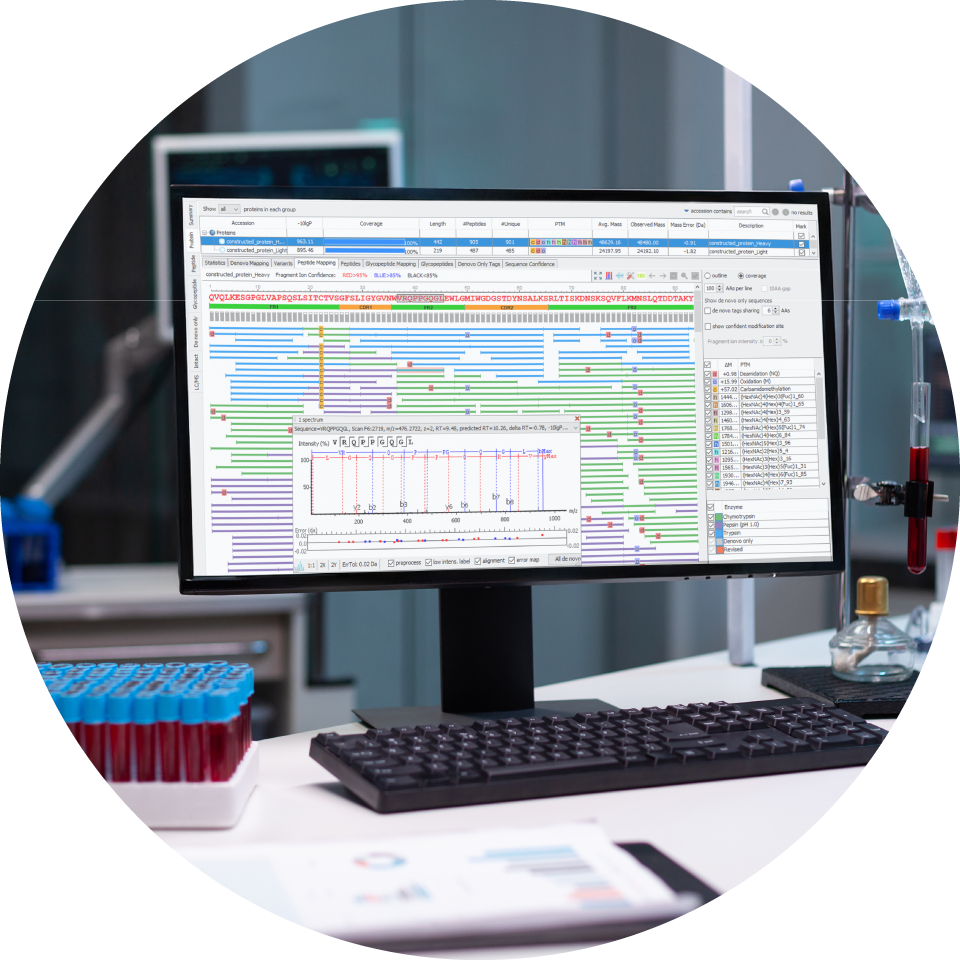
Comprehensive end-to-end workflows from sample preparation to advanced data analysis.

Expert support from PhD scientists – ensuring high-quality results and insightful interpretation.

Fast turnaround times – detailed report and raw data delivered within 1–2 weeks.
Cutting-edge technology – leveraging state-of-the-art PEAKS Software and bioinformatics tools.
Customisable solutions – tailored approaches to meet your specific research needs.
Seamless collaboration – clear communication and dedicated project management.

Request Pricing / Schedule Consultation

We love your feedback!
Service customers receive a 5% discount for their next order for writing us a review, and 10% discount for every referral.

Batch Sample Discounts
Have a big project? We offer discounted pricing for multi-sample orders to ensure an affordable project.

Want to know more? Request more details to learn why our methodology and experience gives you better results than our competitors. Have questions? We’ll be happy to answer any of your inquiries today.





