

PEAKS AB 3.5
Protein de novo sequencing and glycan profiling software
PEAKS AB 3.5 revolutionises protein de novo antibody sequencing with enhanced accuracy, refined PTM (Post-translational modifications) characterisation, and advanced glycan profiling. Key features include retention time prediction for isobaric residues, improved intact mass deconvolution, and enhanced Ile/Leu differentiation. With support for timsTOF data, an upgraded UI, and expanded customisation, PEAKS AB 3.5 sets a new standard in antibody sequencing workflows.
Key Features
- Automated protein de novo sequencing
- Sequence non-antibody proteins with reference sequence
- In-depth glycan profiling
- Enhanced Ile/Leu differentiation
- Quantification of modifications and variants
- QC/QA of antibody production
- Utilise your expertise with manual editing
- Comprehensive report generation
- Supports all major instruments including Orbitrap, timsTOF, zenoTOF
Contact us to add PEAKS AB 3.5 to your lab!
The newest release of PEAKS AB software version 3.5, is equipped with new tools to further improve sequencing accuracy and PTM characterisation. It also provides a more advanced intact mass analysis with increased bottom-up sequencing confidence.
Monoclonal antibodies have a wide range of applications in biochemical analyses, medical research, diagnostics and therapeutics. The protein sequence of an antibody (Ab) encodes critical information for its structure and function. Retrieving the sequence by protein de novo sequencing bypasses the need for screening B-cells, generating hybridoma cell lines or performing next-generation sequencing.
PEAKS AB Software is a platform for de novo antibody sequencing and the characterisation of modifications and sequence variants by using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). PEAKS AB software also supports characterisation of PTMs and variants for research discovery and quality control purposes.
Software Overview
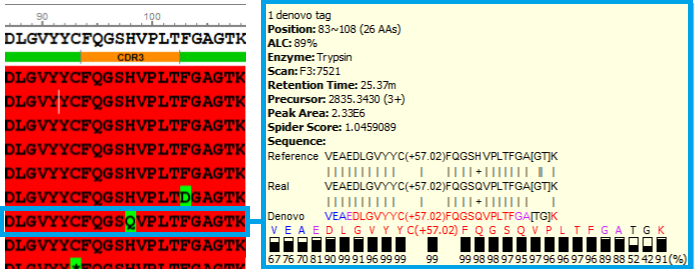
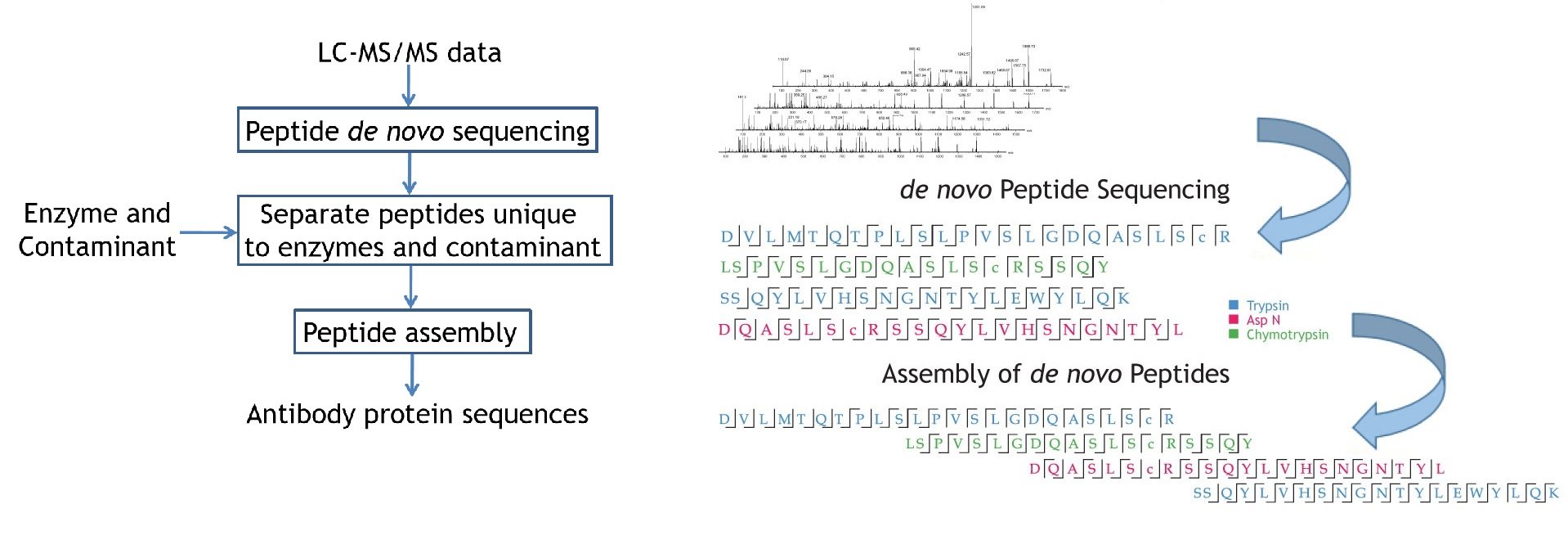
Antibody sequences were obtained from the de novo sequenced peptide. The confident de novo sequence tags, which have direct fragment ion proof, were assembled into antibody sequences. The de novo View shows the assembly of de novo peptide sequence tags.
de novo View
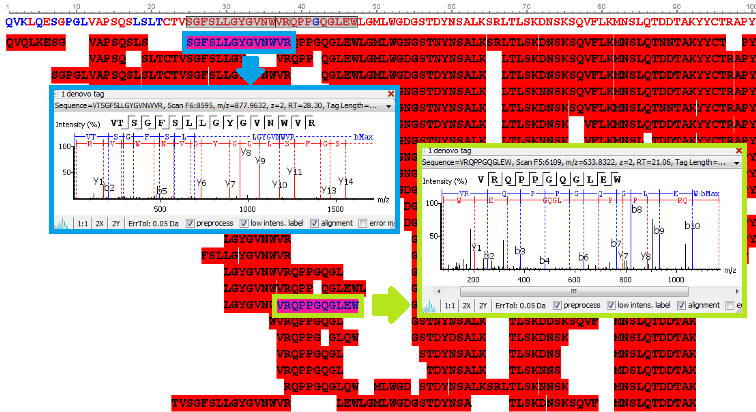
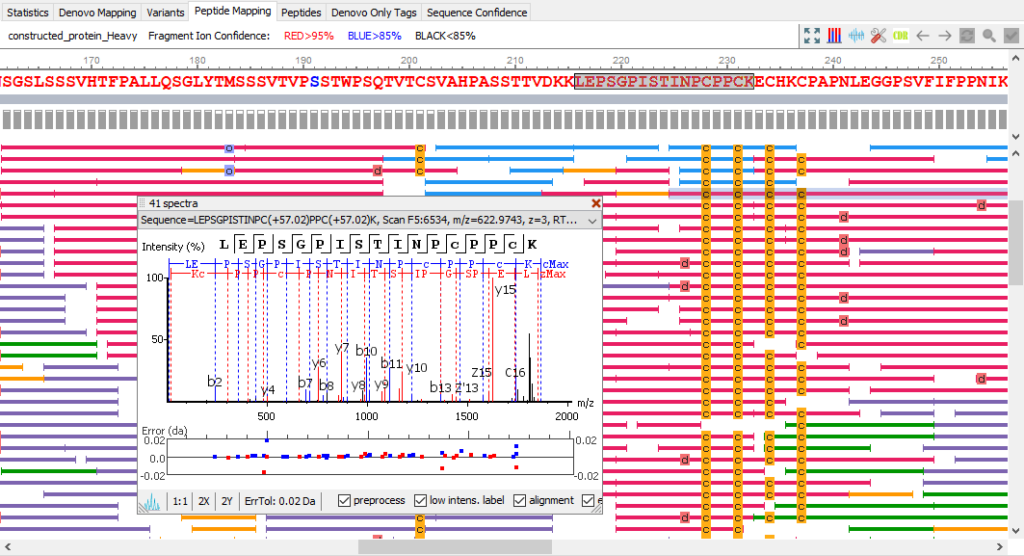
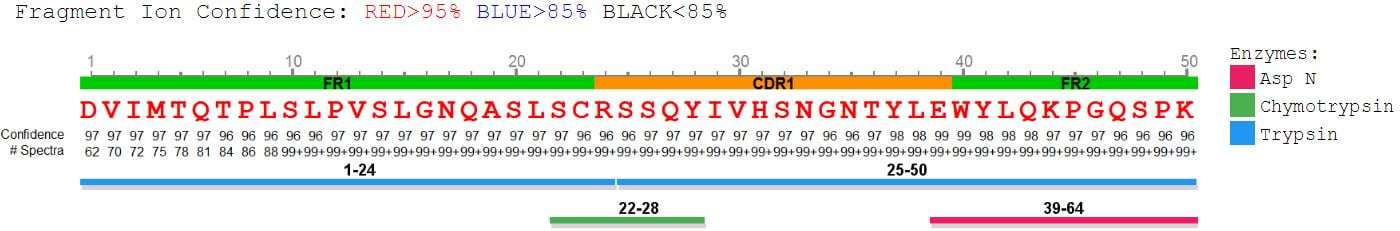
PEAKS AB provides comprehensive peptide mapping, including:
- insight into full sequence information
- displaying each amino acid with confidence from direct fragment ions
- post-translational modifications (PTMs)
- sequence variants
Peptide Mapping View
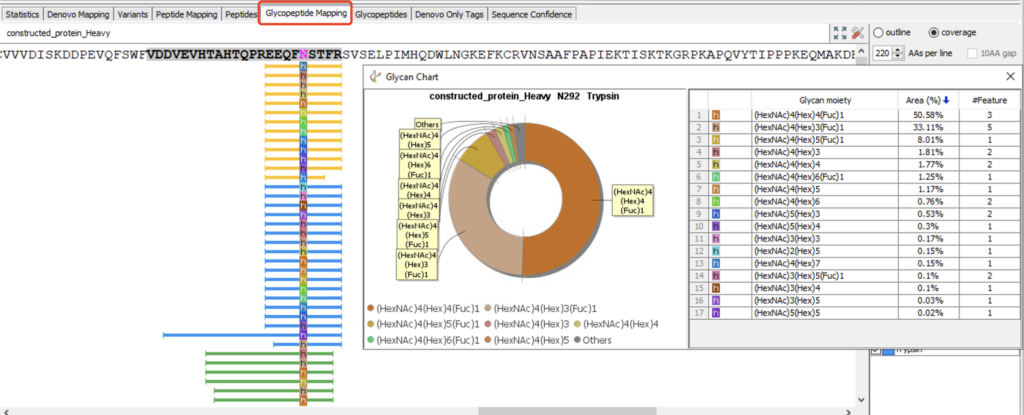
Glycopeptide Mapping View
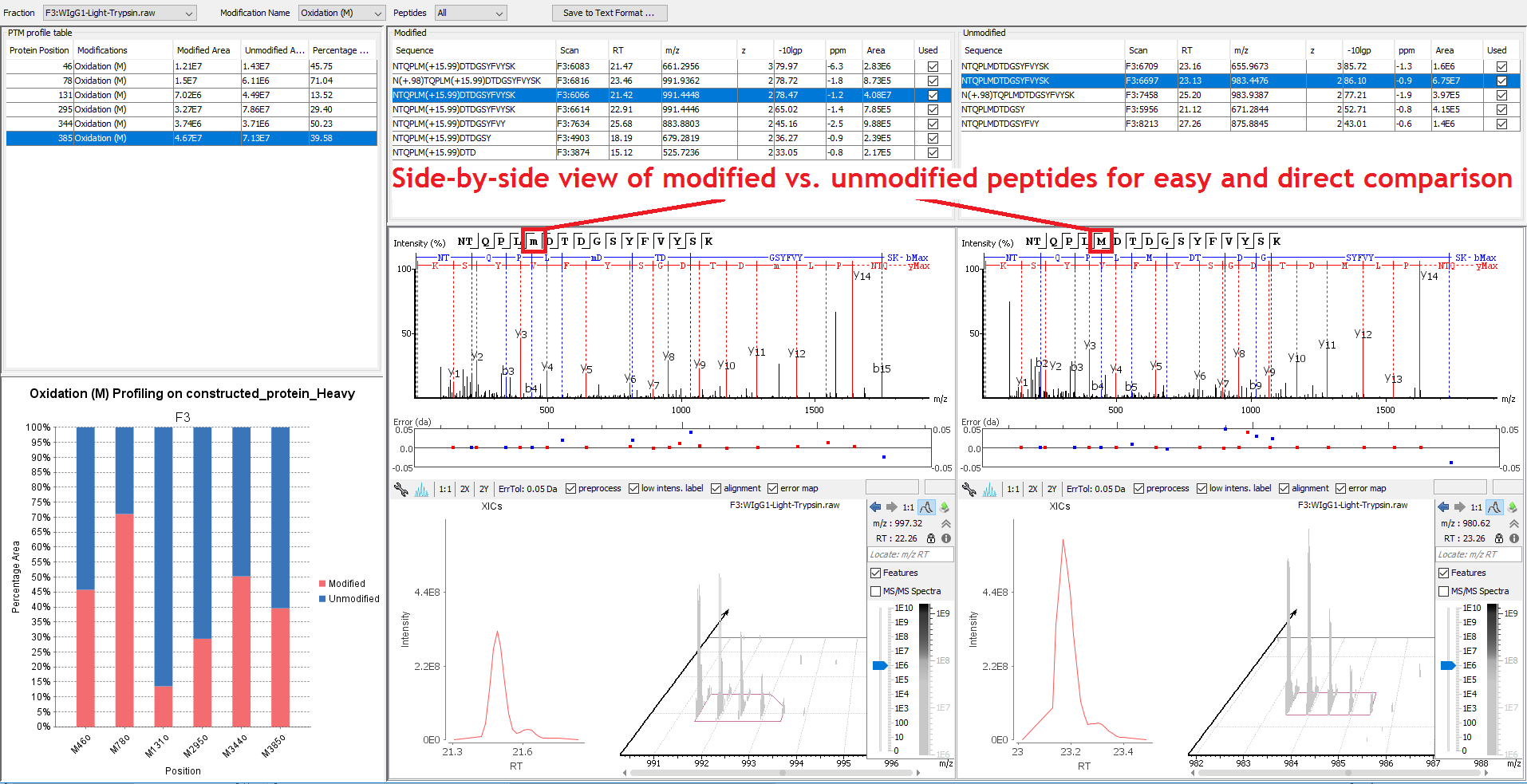
For each sequence position with modifications, the modified and native peptide forms, along with their chromatogram are listed for quantitative viewing.
Post-translational Modifications (PTMs) Analysis
Sequence Variant View highlights amino acid substitutions, insertions, and deletions which may result from erroneous transcription of complementary DNA.
Sequence Variant View
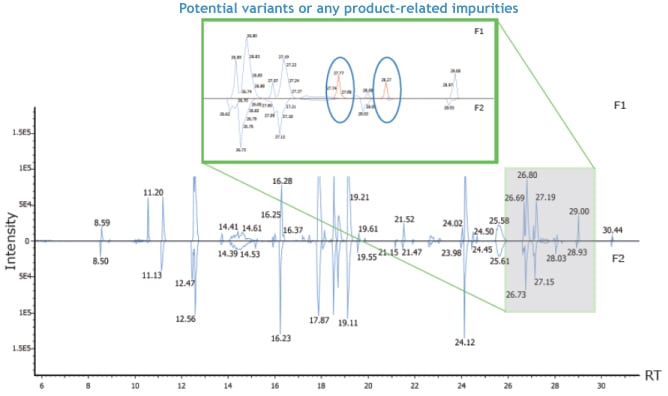
With the enhanced “Peptide Mapping” function, PEAKS AB Software can compare annotated chromatograms between different samples. Possible sequence variants will be illustrated to facilitate the product quality control or any other related antibody studies.
As shown in the image to the right, blue peaks in the annotated chromatogram denote the signals that can be mapped to peptides from reference proteins, and the red peaks denote sequence variants or any product-related impurities.
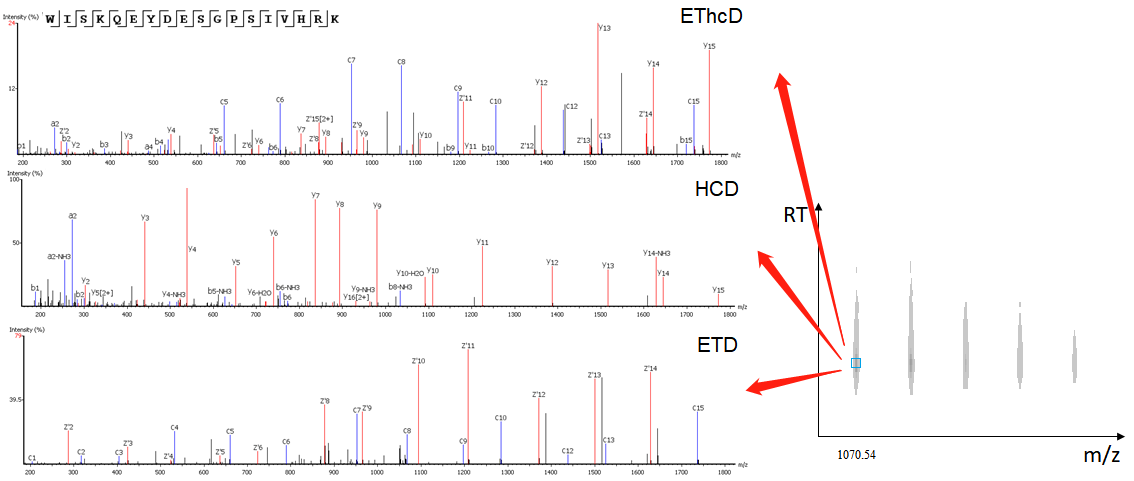
The EThcD fragmentation model was implemented in PEAKS AB 2.0. Moreover, MS2 spectra derived from the same precursor with different types of fragmentations are combined for de novo sequencing, yielding more confident de novo sequencing results.
EThcD Data Analysis
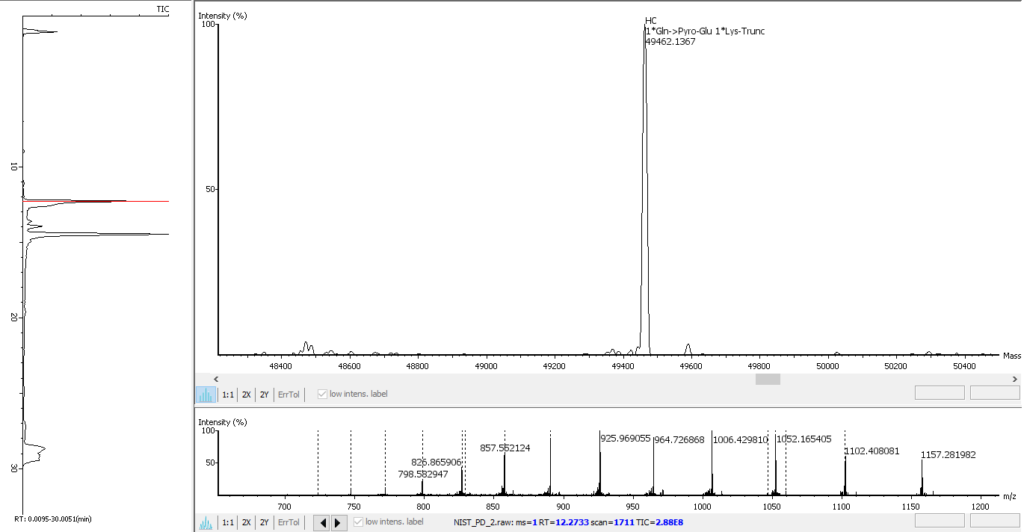
PEAKS AB 3.5 has an improved deconvolution algorithm which enables automated, efficient and accurate intact mass analysis. Intact mass measurements of proteins are performed to validate the assembled sequence and presence of any modifications such as N-terminal pyro-glutamate (Gln -> Pyro-Glu), C-terminal lysine truncation (1*Lys-Trunc) and N-linked glycosylation (`*A2G0F), as shown below. In addition, support from intact mass values have been integrated into the bottom-up peptide mapping result view.
Advanced Intact Mass Deconvolution Analysis
Algorithm
PEAKS AB software directly sequences the antibody using high resolution LC-MS/MS data sets from orthogonal enzyme digests. It performs de novo peptide sequencing first, and assembles the confident sequence tags into antibody sequences.
Report Generation
- Antibody Sample Description
- Abbreviations
- Experimental Procedure
- Antibody Heavy Chain
- Amino Acid Sequence
- Peptide Mapping at 0.1% of FDR at Peptide-Spectrum Level
- Typical Peptides Selected for Variable Region
- Ile/Leu Differentiation
- Antibody Light Chain
- Amino Acid Sequence
- Peptide Mapping at 0.1% of FDR at Peptide-Spectrum Level
- Typical Peptides Selected for Variable Region
- Ile/Leu Differentiation
- Glycan data can be reported using “Generate report” button in Glycan Profiling node.
References & Resources
References
- Shan, B. and Xin, L. Integrating de novo Sequencing and Database Search for Monoclonal Antibody Sequencing. J Biomol Tech. 24(Suppl). S62–S63. 1/5/2013.
- Tran, N.H. et al. Complete De Novo Assembly of Monoclonal Antibody Sequences. Scientific Reports. 6:31730. 26/08/2016.





